Daily Flora Immune
$ 46.00
Size: 30 Capsules
Targeted Probiotic Support for Healthy Nasal, Sinus & Respiratory Function*
Please select all options.
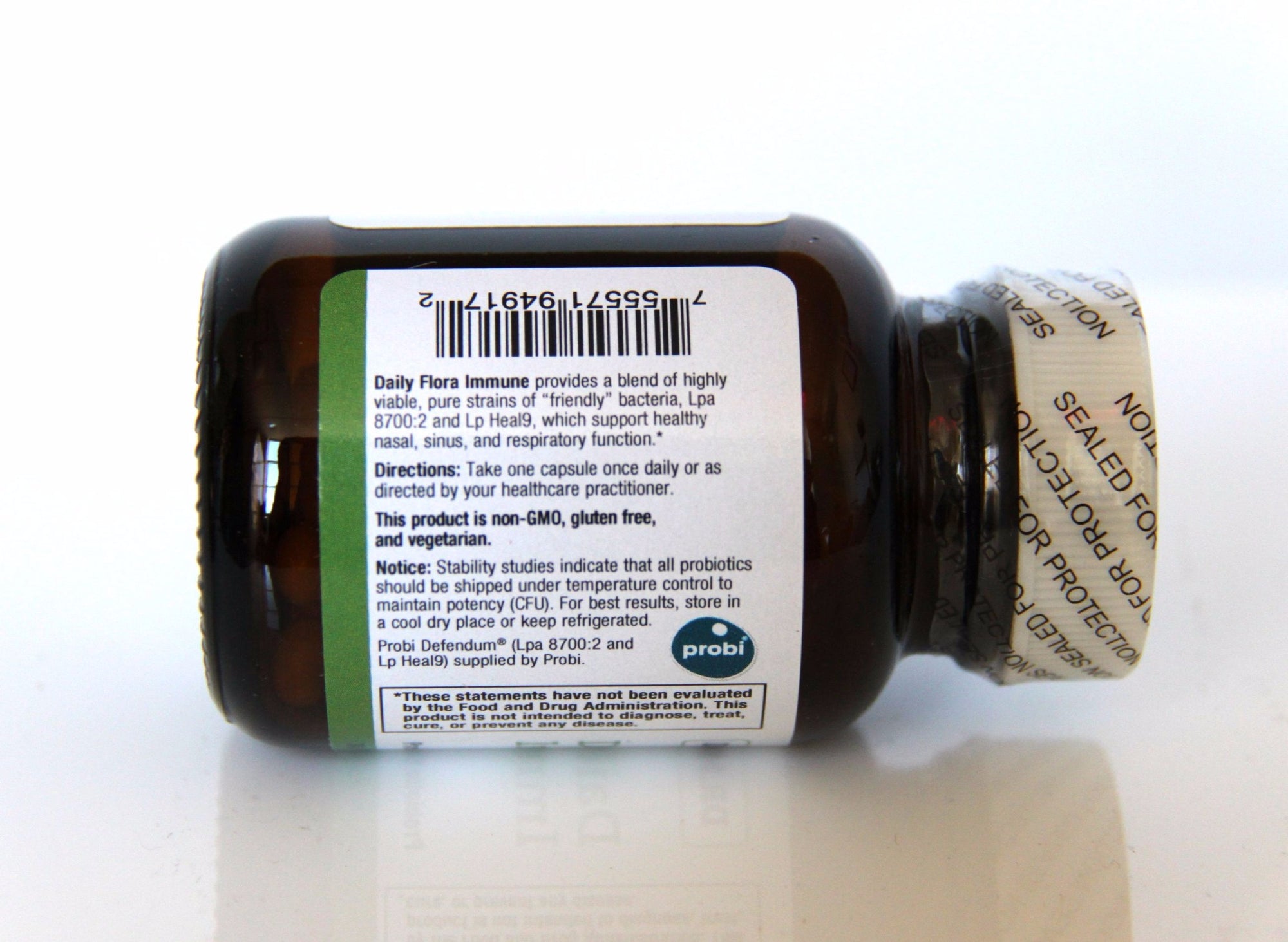
Daily Flora Immune features a research-backed blend of specialized strains of beneficial bacteria known to reduce the risk of upper respiratory tract infections and colds.*[1-4] These two highly-viable Lactobacillus strains—L. plantarum HEAL9 and L. paracasei 8700:2—have been shown in numerous clinical studies to reduce the incidence of colds and other viral infections in both children and adults.*
Are you always getting the sniffles during the fall and winter seasons? Kids bringing home a cough from school? We’ve seen that the studies are right—the potent probiotic combo in Daily Flora Immune is the perfect addition to your everyday routine to boost your immune system and support your upper respiratory health.*
*These statements have not been evaluated by the FDA and are not intended to treat or cure any disease.
When it comes to probiotics, not all bacteria are equal! Only some beneficial bacteria can survive through the harsh stomach and replicate in the gastrointestinal tract to have a full therapeutic effect. And, different bacteria have different effects. The only way to know which bacteria have the benefits we want is through research, which is why Dr. Morrison uses two proven strains backed by extensive clinical research in Daily Flora Immune.
It isn’t just the species that matters, it’s the strain! Daily Flora Immune uses the HEAL9 strain of Lactobacillus plantarum and the 8700:2 strain of Lactobacillus paracasei—the specific strains from clinical studies. Research shows that not all strains of the same species have similar actions,5 and some strains of L. plantarum and L. paracasei are completely inactive!6 Think about it like dogs—all dogs are from the same species (Canis familiaris), but you wouldn’t try to use a chihuahua to herd sheep!
1 CAPSULE PER SERVING
A Proprietary Probiotic Blend 37.6 mg(1 billion CFU) of:
Lactobacillus paracasei 8700:2
Lactobacillus plantarum HEAL9
Other Ingredients: Starch, capsule (hypromellose, gellan gum), and magnesium stearate (vegetable)
Vegetarian capsule
Take one capsule once daily or as directed by your healthcare practitioner.
Storage: No refrigeration required. Store below 75º
Background: The aim of this study was to investigate whether consumption of Lactobacillus plantarum HEAL 9 (DSM 15312) and Lactobacillus paracasei 8700:2 (DSM 13434) could affect naturally acquired common cold infections in healthy subjects.
Methods: A randomised, parallel, double-blind placebo-controlled study was performed to investigate whether intake of this probiotic mixture could reduce the risk of common cold episodes, number of days with common cold symptoms, frequency and severity of symptoms, and cellular immune response in common cold infections. A total of 272 subjects were supplemented daily with either 10(9) cfu (colony forming units) of probiotics (N = 135) or control (N = 137) for a 12-week period.
Results: The incidence of acquiring one or more common cold episode was reduced from 67% in the control group to 55% in the probiotic group (p < 0.05). Also, the number of days with common cold symptoms were significantly (p < 0.05) reduced from 8.6 days in the control group to 6.2 days, in the probiotic group, during the 12-week period. The total symptom score was reduced during the study period from a mean of 44.4 for the control group to 33.6 for the probiotic group. The reduction in pharyngeal symptoms was significant (p < 0.05). In addition, the proliferation of B lymphocytes was significantly counteracted in the probiotic group (p < 0.05) in comparison with the control group.
Conclusion: In conclusion, intake of the probiotic strains Lactobacillus plantarum HEAL 9 (DSM 15312) and Lactobacillus paracasei 8700:2 (DSM 13434) reduces the risk of acquiring common cold infections.
Background: The combination of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 (commercially available as Probi Defendum®) has previously been reported to reduce the incidence, duration and severity of naturally acquired common colds in adults. The aim of the present study was to evaluate the impact of Probi Defendum® on aspects of common cold in healthy children 1-6 years of age attending day care.
Methods: A total of 131 children, out of the planned 320, were recruited into the study during 1 common cold season and randomised to consume once daily either 109 CFU (colony forming units) of the probiotic product or placebo. Due to unforeseen reasons, the recruitment of more children did not continue beyond the first cold season.
Results: There were 106 children that completed the study out of the 131 randomised. Daily consumption of the probiotic product for a period of 3 months significantly reduced the severity of the symptom "nasal congestion/runny nose" with a mean severity score for the whole study period of 7.5 ± 9.7 in the probiotic group and 13.9 ± 15.2 in the placebo (p < 0.05). Moreover, significantly less concomitant medication was used in the probiotic group. When the data were projected to a larger population corresponding to the originally estimated sample size, the results were in favour of the probiotic group regarding the reduced absence from day care (p < 0.05), reduced mean total severity per day in the reported episodes (p < 0.05) and reduced severity of the symptom "crying more than usual" (p < 0.05).
Conclusion: Intake of Probi Defendum® once daily for a period of 3 months was beneficial to children and reduced the severity of common colds.